Background: Despite recent therapeutic advances, an unmet need exists for efficacious, well-tolerated, and convenient treatment (tx) options for patients (pts) with relapsed/refractory (R/R) follicular lymphoma (FL). In particular, pts with high-risk disease, including those refractory to both anti-CD20 tx and an alkylating agent (double refractory) and those with disease progression within 2 y of first-line (1L) immunochemotherapy (POD24), require more effective options. Epcoritamab, a subcutaneous (SC) CD3xCD20 bispecific antibody, was recently approved by the US FDA for the tx of adults with R/R diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from indolent lymphoma, and high-grade B-cell lymphoma after ≥2 lines of systemic tx. Here we present initial results from the FL dose-expansion cohort of the EPCORE™ NHL-1 trial (NCT03625037; phase 1/2).
Methods: Pts with CD20 + R/R FL (grade [G] 1-3A) who had received ≥2 prior lines of systemic tx received epcoritamab SC in step-up doses (SUD 1 and 2) in cycle (C) 1, followed by full doses of 48 mg in 28-d Cs: QW, C1-3; Q2W, C4-9; and Q4W, C≥10 until disease progression or unacceptable toxicity. The primary endpoint of overall response rate (ORR) was assessed per Lugano criteria by an independent review committee. As a secondary analysis, minimal residual disease (MRD) was assessed in peripheral blood using the clonoSEQ ® assay (Adaptive Biotechnologies, Seattle, WA).
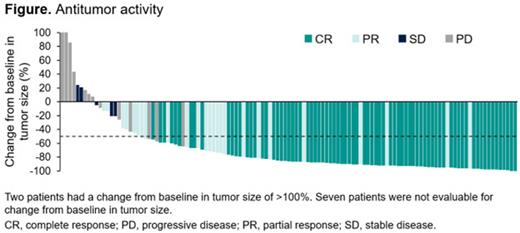
Results: Between Sep 2020 and Oct 2022, 128 pts with R/R FL G1-3A were enrolled to receive epcoritamab SC. As of Apr 21, 2023, the median follow-up was 17.4 mo. Median age was 65 y, 61% of pts had FLIPI 3-5, and 85% had stage III-IV disease. The median number of prior lines of tx was 3 (range, 2-9); 31% of pts had ≥4 prior lines of tx. Common prior therapies included anthracyclines (77%), lenalidomide (31%), and autologous stem cell transplant (19%). Most pts were primary refractory (54%), double refractory (70%), or refractory to their last prior tx (69%); 42% had POD24, and 52% progressed within 2 y of initiating any 1L tx. The ORR was 82%, with a complete response (CR) rate of 63% ( Figure). The median time to response and CR was 1.4 and 1.5 mo, respectively. ORRs/CR rates were generally consistent across prespecified high-risk subgroups: double refractory, 76%/56%; refractory to last prior tx, 74%/51%; POD24, 80%/61%; progression within 2 y of initiating any 1L tx, 79%/64%. High ORRs/CR rates were observed across lines of tx, with a trend of higher rates in earlier lines: 2 prior lines, 89%/72%; 3 prior lines, 88%/68%; ≥4 prior lines, 68%/45%. Median progression-free survival (PFS) was 15.4 mo; median duration of response, duration of CR, and overall survival were not reached. An estimated 85% and 74% of pts with CR remained in response at 12 and 18 mo, respectively. MRD negativity was correlated with improved PFS. The most common tx-emergent AEs (TEAEs) of any grade were CRS (66%), injection-site reaction (57%), COVID-19 (40%), fatigue (30%), neutropenia (28%), diarrhea (27%), and pyrexia (25%). TEAEs leading to tx discontinuation occurred in 19% of pts, the most common being COVID-19. CRS was mostly low grade (40% G1, 25% G2, 2% G3) and primarily occurred following the first full dose (median time to onset after first full dose, 15 h). No CRS events led to tx discontinuation. ICANS was reported in 8 pts (6% overall; 4% G1, 2% G2); all resolved without leading to tx discontinuation. Fatal TEAEs occurred in 13 pts (10%). In a separate R/R FL cohort evaluating an optimized SUD regimen in C1 to mitigate CRS risk, a clinically meaningful reduction in CRS incidence and severity was observed (data to be presented).
Conclusions: Single-agent epcoritamab SC resulted in deep and durable responses with high ORR and CR rates in hard-to-treat, high-risk pts with R/R FL. Responses were consistent across subgroups. A correlation between MRD negativity and PFS was observed. The safety profile was manageable; CRS occurrence was predictable, and a reduction in CRS occurrence and severity was seen with an optimized SUD regimen. Overall, no new safety signals were detected. Epcoritamab is currently being investigated in several FL trials across lines of tx, including an ongoing phase 3 trial (NCT05409066) in R/R FL.
Disclosures
Linton:Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Hoffman-La Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; Roche: Consultancy; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Jurczak:BeiGene: Consultancy; AbbVie: Consultancy; AstraZeneca: Consultancy; Eli Lilly: Consultancy; Pfizer: Consultancy; Roche: Consultancy; SOBI: Consultancy; Takeda: Consultancy; AbbVie: Research Funding; AstraZeneca: Research Funding; Bayer: Research Funding; BeiGene: Research Funding; Celgene: Research Funding; Janssen: Research Funding; Eli Lilly: Research Funding; Merck: Research Funding; Pfizer: Research Funding; Roche: Research Funding; SOBI: Research Funding; Takeda: Research Funding. Gyan:AbbVie: Other: Congress travel fee and hospitality; Amgen: Other: Congress travel fee and hospitality; Alexion: Honoraria; Astellas: Other: Coordinating investigator for industry sponsored studies; BMS: Honoraria; Eusa Pharma: Honoraria; Gilead: Honoraria, Other: Congress travel fee and hospitality; Jazz Pharma: Honoraria; MSD: Research Funding; Pfizer: Honoraria; Pharmacyclics: Other: Coordinating investigator for industry sponsored studies; Novartis: Honoraria, Research Funding; Roche: Honoraria, Other: Congress travel fee and hospitality. Coordinating investigator for industry sponsored studies; Sandoz: Honoraria, Research Funding; Sanofi: Honoraria, Other: Congress travel fee and hospitality, Research Funding. Sureda Balari:Gilead: Consultancy; Sanofi: Consultancy, Honoraria; Gilead Kite: Honoraria; Amgen: Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; MSD: Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria; Alexion: Honoraria. Hess:Bristol Myers Squibb: Consultancy; ADC Therapeutics: Consultancy. Tilly:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees. Cordoba:F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Consultancy; F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Speakers Bureau; European Hematology Association (EHA), Spanish Society Hematology (SEHH): Membership on an entity's Board of Directors or advisory committees; Fundacion Jimenez Diaz University Hospital: Current Employment. Lewis:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hutchings:AbbVie: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Incyte: Research Funding; Novartis: Research Funding. Clausen:Aztrazenica: Other: advisory; Incyte: Consultancy; Abbvie: Consultancy, Other: advisory; Janssen: Consultancy, Other: advisory; Roche: Other: Travel funding; Genmab: Consultancy, Other: advisory; Gilead: Consultancy. Vitolo:Servier: Other: Lecture Fees; Roche: Other: Lecture Fees; Janssen: Other: Lecture Fees; Incyte: Other: Lecture Fees; AbbVie: Other: Lecture Fees; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees. Cochrane:Janssen-Cilag: Speakers Bureau; Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Beigene: Research Funding. Leppa:Abbvie, Beigene, Gilead, Incyte, Novartis, Orion, Pfizer, F. Hoffmann-La Roche Ltd, Sobi: Consultancy; Bayer, Celgene, Genmab, Hutchmed, Novartis, Nordic Nanovector, F. Hoffmann-La Roche Ltd, (Research Funding to the Institute): Research Funding; Gilead, F. Hoffmann-La Roche Ltd, Novartis, Incyte: Honoraria. Chamuleau:Abbvie: Consultancy; Novartis: Consultancy; Sanofi: Consultancy; BMS/Celgene: Research Funding; Gilead: Research Funding; Genmab: Research Funding. Conlon:AbbVie: Current Employment. Favaro:Genmab: Current Employment. Gernhardt:Genmab: Current Employment. Altintas:Genmab: Current Employment, Current equity holder in publicly-traded company. Liu:Genmab: Current Employment. Thieblemont:Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Kyte, Gilead, Novartis, BMS, Abbvie, F. Hoffmann-La Roche Ltd, Amgen: Honoraria; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Janssen: Honoraria, Other: Travel Expenses; Hospira: Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria; Paris University, Assistance Publique, hopitaux de Paris (APHP): Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal